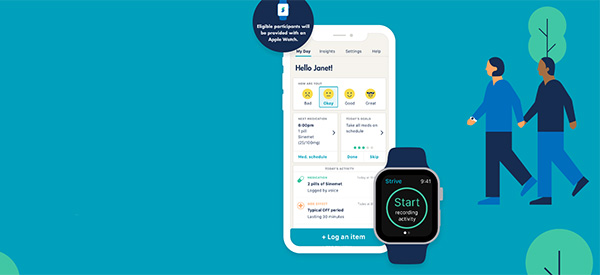
The Food and Drug Administration (FDA) has granted 510(k) clearance to Rune Labs’ StrivePD Ecosystem for monitoring Parkinson’s disease through the Apple Watch.
Rune Labs is a precision neurology platform that focuses vastly on exploring new techniques of care delivery and data-driven routine care. The StrivePD Ecosystem app will monitor Parkinson’s disease symptoms as well as treatment outcomes, while also advancing clinical trials for new Parkinson’s therapeutics. The app makes use of motion sensors integrated into the Apple Watch, which can already detect when someone falls.
The FDA approval of Rune Labs’ application advances the use of software for analyzing movement disorders. With the approval, tens of thousands of Parkinson’s sufferers who currently use these gadgets in their everyday lives will take part in the trial. Consequently, approval also paves the way for StrivePD to reach a large number of possible prodromal Parkinson’s patients.
Furthermore, easy access to the StrivePD app via Apple Watch allows Parkinson’s patients to document and track their symptoms, giving them more autonomy over their health care.
Prior to the advancement of medical technology, physicians had to make decisions and treatment methods based on limited data available through patient care support or during short clinical visits. But, with StrivePD, a multimodal data will be generated, which will essentially transform the way people with movement disorders—particularly Parkinson’s patients—are treated.
The new ecosystem will offer physicians with a continual stream of observations over lengthy periods of time, allowing them to better understand the disease and how it works.


But how do you calibrate it correcty so it wouldnt trigger if you lay down too quickly. Or if you are doing push ups?
Andrew, what we know is in the article. If you have questions, I’d recommend that you contact Rune Labs with your questions; there’s a link in the article.
Alfred Poor, Editor
Health Tech Insider