Mobile devices in general — and wearable devices in particular — depend heavily on rechargeable batteries to provide the electricity that they need to operate. And the technology of choice these days is lithium-ion which has good storage capacity and reliability, though it does have occasional problems with run-away reactions that can lead to fires. Alkaline batteries are safer, but generally are not rechargeable. Researchers at Stanford University are working on a new approach that could surpass both incumbent designs.
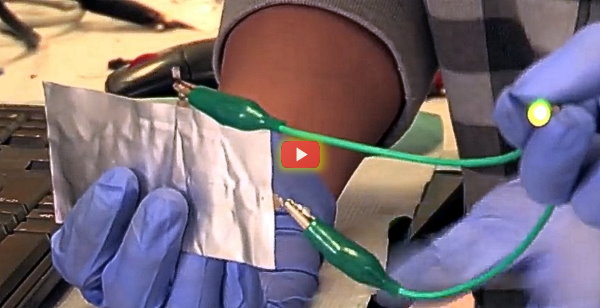
The battery is made up of aluminum and graphite (carbon), two of the most common and inexpensive materials available. It also uses a liquid salt electrolyte, with the whole device encased in a plastic pouch. The result is a device that is thin, lightweight, and as can be seen in the video above, very flexible. You can poke a hole through the battery and it will not explode or catch fire. And perhaps the most appealing feature of all is that can be recharged in just one minute.
The battery puts out enough power to be useful; a pair of the modules can power a typical smartphone. The current version has an energy density equivalent to a lead-acid car battery, but the scientists see paths to improve that capacity. The prototype has already been able to withstand more than 7,500 recharge cycles without any loss in capacity; most lithium-ion batteries are limited to only about 1,000 cycles. It will take time before this technology can make it out of the lab and into commercial production, but its combination of low cost materials, flexibility, fast charging, and safe design should make it an attractive target for industrial development.