Diabetes is a global pandemic afflicting more than 420 million people, according to the Word Health Organization (WHO). Diabetics face a lifetime of checking blood glucose levels and calculating and injecting the necessary amount of insulin. Artificial pancreas systems such as the Medtronic iPro2, consisting of continuous glucose monitoring and automatic insulin delivery, are just entering the market. Separate glucose monitors and insulin pumps are more prevalent. Most continuous glucose monitors work for a limited time and must be calibrated or checked with physical blood tests daily.
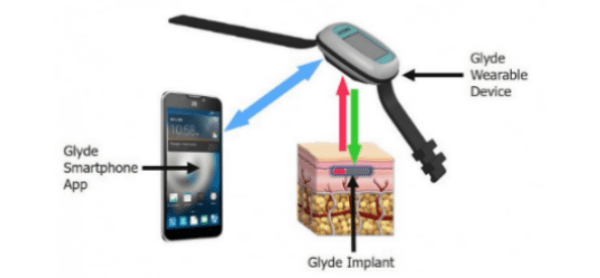
GluSense is focused on the development of a long-term, injectable, continuous glucose sensor. The GluSense Glyde system includes an implanted sensor, a wearable device that reads and calibrates glucose data received from the implant, and a smartphone app that displays and stores the readings. The Senseonics Eversense system has a similar 3-piece configuration, sensing glucose with a fluorescent protein biosensor in the implant. The Eversense implant must be replaced every 90 days because the active material decays. The Glyde design is unique in that the biosensor is replenished by modified live cells encapsulated inside the device that create the biosensor material. The minimum duration target for the Glyde implant is one year. The firm is also intent on setting a standard for accuracy such that patients won’t have to supplement the CGM with conventional finger stick tests.
According to the GluSense website FAQ, the product is several years away from availability, but they hope to start clinical studies soon. Diabetics and their care teams are cautious in using artificial pancreas systems and continuous glucose monitors because of the ramifications of malfunction or miscalculation. The science applied to various solution candidates by different companies heighten the prospect of one or more reliable and safe long-term management systems for the world’s diabetics.

